Background: Results with CD19-directed CAR T cell therapy in patients (pts) with second-line (2L) R/R follicular lymphoma (FL) and high-risk features, such as progression of disease within 24 months (POD24) from diagnosis or double refractory to anti-CD20 antibody plus alkylator, have not been previously reported. TRANSCEND FL (NCT04245839), a global, phase 2, open-label, single-arm, multicohort, pivotal study, assessed efficacy and safety of the anti-CD19 CAR T cell therapy lisocabtagene maraleucel (liso-cel) in pts with second line or later (2L+) R/R indolent NHL. Some data from the primary analysis were previously reported, including safety in 2L+ R/R FL, and focused on efficacy in third line or later R/R FL (Morschhauser F, et al. Hematol Oncol 2023;41[S2]:877‒880). Here, we report primary analysis results in the cohort of pts with 2L high-risk R/R FL.
Methods: Eligible pts in the 2L R/R FL cohort had biopsy-confirmed FL before enrollment and must have had POD24 with treatment ≤ 6 months from original FL diagnosis and/or must have had high tumor burden as defined by modified Groupe d'Etude des Lymphomes Folliculaires (mGELF) criteria. All pts received 1 prior combination systemic therapy with an anti-CD20 antibody and alkylator. Eligible pts received liso-cel (100 × 10 6 CAR + T cells) after lymphodepleting chemotherapy (LDC). Bridging therapy was allowed with reconfirmation of PET-positive disease before LDC. The primary endpoint was ORR per independent review committee (IRC) by PET/CT using Lugano 2014 criteria. Secondary endpoints included CR rate, duration of response (DOR), PFS, OS, safety, and cellular kinetics. Pharmacodynamic endpoints were exploratory.
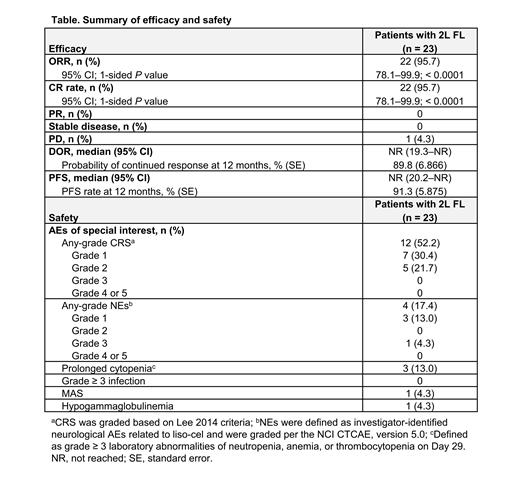
Results: At data cutoff (January 27, 2023), 23 of 25 leukapheresed pts received liso-cel and were evaluable for safety and efficacy per IRC; 1 received nonconforming product and 1 reached CR after bridging therapy and no longer met eligibility criteria. Median (range) age was 53 y (34-69), 74% had stage III/IV disease, and 35% were high-risk per FL International Prognostic Index (FLIPI). Sixty-five percent of pts had POD24 from initiation of first-line combination chemoimmunotherapy (52% had POD24 from diagnosis), 70% met mGELF criteria (mGELF only, 48%; mGELF and POD24 from diagnosis, 22%), and 48% were double refractory to anti-CD20 antibody plus alkylator. Median (range) on-study follow-up was 18.1 months (1.0-26.8). In efficacy-evaluable pts, the ORR and CR rate were both 95.7% (95% CI, 78.1-99.9; 1-sided P < 0.0001; Table).
With a median follow-up of 16.8 months and 17.8 months, respectively, median DOR and PFS were not reached; 12-month DOR and PFS were 89.8% and 91.3%, respectively. The most common grade (gr) ≥ 3 treatment-emergent AEs (TEAE) were cytopenias; neutropenia was most frequent (52%). Cytokine release syndrome (CRS) occurred in 12 (52%) pts (no gr ≥ 3). Median (range) time to onset and resolution of CRS was 6 days (2-9) and 3 days (2-7), respectively. Neurological events (NE) occurred in 4 (17%) pts, with 1 (4%) gr 3 and no gr 4-5 (Table). Median (range) time to onset and resolution of NEs was 8.5 days (6-11) and 2.5 days (1-4), respectively. Three (13%) pts received tocilizumab/steroids for CRS/NEs. Prolonged cytopenia (gr ≥ 3 laboratory values at Day 29) occurred in 3 (13%) pts; all recovered to gr ≤ 2 by Day 90. No gr ≥ 3 infections were reported. One TEAE death occurred in the context of IRC-assessed disease progression due to gr 5 macrophage activation syndrome (MAS). Liso-cel showed rapid expansion with median (range) time to maximum transgene levels of 10 days (7-11). Persistence of liso-cel transgene was detected up to Month 12 in 5 of 18 (28%) pts. B-cell aplasia (< 3% CD19 + B cells in peripheral blood lymphocytes) after liso-cel infusion was rapid and maintained in ≥ 95% of pts through Month 2.
Conclusions: This is the first report of outcomes in 2L high-risk R/R FL with CD19-directed CAR T cell therapy. In this population, liso-cel achieved very high CR rates (22 of 23 pts); deep and durable remissions, with follow-up ongoing; and a favorable safety profile with low rates of severe (gr ≥ 3) CRS, NEs, and prolonged cytopenia, and no severe infections. These data support liso-cel as a potential new treatment option in pts with 2L R/R FL at high-risk for treatment failure.
Disclosures
Morschhauser:Janssen: Honoraria; Gilead: Consultancy, Other: Advisory Board; BMS: Consultancy, Other: Advisory Board; AbbVie: Consultancy, Other: Advisory Board; Celgene: Other: Advisory Board; Novartis: Consultancy, Other: Advisory Board; Incyte: Other: Advisory Board; Epizyme: Other: Advisory Board; Genmab: Consultancy, Other: Advisory Board; Roche: Consultancy, Honoraria, Other: Advisory Board. Dahiya:Adaptive Biotechnologies: Consultancy; Bristol Myers Squibb: Consultancy; Incyte: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding. Palomba:Rheos: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Smart Immune: Honoraria; Thymofox: Honoraria; Ceramedix: Honoraria; BMS: Honoraria; Juno: Honoraria, Patents & Royalties; Cellectar: Honoraria; Novartis: Honoraria; MustangBio: Honoraria; Pluto Immunotherapeutics: Honoraria; Kite: Honoraria; GarudaTherapeutics: Honoraria; Synthekine: Honoraria. Martin Garcia-Sancho:Kyowa Kirin: Consultancy, Honoraria; Clinigen: Consultancy; Eusa Pharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Gilead / Kite: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; ADC Therapeutics America: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Ideogen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd, BMS / Celgene, Kyowa Kirin, Novartis, Gilead / Kite, Incyte, Lilly, ADC Therapeutics America, Miltenyi, Ideogen, Abbvie, Sobi: Consultancy; F. Hoffmann-La Roche Ltd, BMS/Celgene, Janssen, Gilead/Kite, Takeda, Eusa Pharma, Abbvie: Honoraria; Roche: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Reguera:BMS: Speakers Bureau; KITE: Speakers Bureau; AMGEN: Speakers Bureau; Janssen: Consultancy, Speakers Bureau. Kuruvilla:Abbvie, BMS, Gilead, Merck, Roche, Seattle Genetics: Consultancy; Abbvie, Amgen, Astra Zeneca, BMS, Genmab, Gilead, Incyte, Janssen, Merck, Novartis, Pfizer, Roche, Seattle Genetics: Honoraria; Roche, Astra Zeneca, Merck: Research Funding; Karyopharm: Other: DSMB. Jaeger:Innovative Medicines Initiative 2 Joint Undertaking: Research Funding; BMS, Novartis, Gilead, Miltenyi, Janssen and Roche: Honoraria. Cartron:MabQi: Consultancy; MedxCell: Consultancy; Janssen: Honoraria; Novartis: Honoraria; Gilead: Honoraria; Emercell: Consultancy; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Jansen, Gilead, Novartis, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Honoraria; MedxCell, Ownards Therapeutics, MabQi, Emercell, F. Hoffmann-La Roche Ltd, BMS, Abbvie: Consultancy; MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Ownards Therapeutics: Consultancy; Roche: Consultancy, Honoraria. Izutsu:Nihon Kayaku: Honoraria; Meiji Seika: Honoraria; Eli Lilly: Honoraria; SymBio Pharmaceuticals: Honoraria; Janssen: Honoraria; Regeneron: Research Funding; Loxo Oncology: Research Funding; Beigene: Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Yakult: Research Funding; Novartis: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Incyte: Research Funding; Astellas Amgen: Research Funding; Nippon Shinyaku: Consultancy; Mitsubishi Tanabe Pharma: Consultancy; Zenyaku Kogyo: Consultancy; Kyowa Kirin: Honoraria, Research Funding; Chugai Pharma: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; MSD: Honoraria, Research Funding; Genmab: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Ono Pharmaceuticals: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Eisai: Consultancy, Honoraria, Research Funding; Otsuka: Consultancy, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Dreyling:Astra Zeneca, Beigene, Gilead/Kite, Janssen, Lilly, Novartis, Roche: Honoraria; Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche: Research Funding; Abbvie, Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche: Other: Scientific advisory boards. Ghesquieres:Gilead, Roche, BMS, Abbvie: Honoraria; Gilead, Roche: Consultancy. Ardeshna:Novartis, Gilead, Bristol Myers Squibb: Other: travel support. Goto:SymBio: Research Funding; Bristol-Myers Squibb: Research Funding; Novartis: Honoraria; Chugai: Honoraria; Kyowa Kirin: Honoraria, Research Funding. Abramson:Novartis: Consultancy; Celgene: Consultancy; Takeda: Consultancy; Seagen Inc.: Research Funding; Regeneron: Consultancy, Honoraria; Ono Pharma: Consultancy; Mustang Bio: Consultancy, Research Funding; MorphoSys: Consultancy; Merck: Research Funding; Lilly: Consultancy; Kymera: Consultancy; Kite Pharma: Consultancy; Janssen: Consultancy, Honoraria; Interius: Consultancy; Incyte: Consultancy; Genmab: Consultancy; Genentech: Consultancy; Epizyme: Consultancy; Century Therapeutics: Consultancy; EMD Serono: Consultancy; Cellectar Biosciences: Consultancy; Caribou Biosciences: Consultancy; BMS: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy; AstraZeneca: Consultancy, Honoraria; AbbVie: Consultancy; Alimera Sciences: Consultancy; Karyopharm Therapeutics: Consultancy; C4 Therapeutics: Consultancy; Bluebird Bio: Consultancy; AI Therapeutics: Research Funding. Borchmann:BMS Germany; MSD Oncology: Honoraria; MSD Oncology; Takeda: Research Funding. Fleury:Abbvie, Astrazeneca, Beigene, Janssen, Roche: Consultancy; Abbvie, Astrazeneca, Bristol Myers Squibb, Gilead, Janssen, Merck, Novartis, Roche, Seattle Genetics: Consultancy, Other: Advisory board; BMS: Consultancy; Incyte, Gilead, Novartis, Seagen: Consultancy, Speakers Bureau. Mielke:SWECARNET: Other: Founder/Leadership (via my institution) ; ScientifyResearch: Other: Founder (spouse) ; Immunicum/Mendes, Miltenyi: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Celgene/BMS, Novartis, Janssen, Gilead/KITE, JSMO, Pfizer: Speakers Bureau. Skarbnik:Abbvie: Consultancy, Honoraria, Speakers Bureau; Alexion: Consultancy, Honoraria; Genentech, Inc.: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Speakers Bureau; Genmab: Consultancy, Honoraria, Speakers Bureau; Epizyme: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria, Speakers Bureau; Lilly: Consultancy, Honoraria, Speakers Bureau; MorphoSys: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; SeaGen: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers-Squibb: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Beigene: Honoraria, Speakers Bureau; ADC therapeutics: Honoraria, Speakers Bureau. de Vos:BeiGene: Consultancy. Kamdar:Adaptive Biotechnologies: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Novartis: Research Funding; syncopation: Consultancy; Genentech: Consultancy; Beigene: Consultancy; ADC therapeutics: Consultancy; caribou biosciences: Consultancy; SeaGen: Speakers Bureau; Celgene: Other: DMC; Genentech: Other: DMC. Karmali:BMS: Consultancy, Honoraria, Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Lilly: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria; Takeda: Research Funding; Calithera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Miltenyi: Consultancy, Honoraria, Research Funding; Morphosys: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy. Viardot:F. Hoffmann-La Roche Ltd, Abbvie, Kite/Gilead, BMS: Consultancy; F. Hoffmann-La Roche Ltd, Abbvie, Kite/Gilead, BMS: Honoraria; BMS: Research Funding. Farazi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Fasan:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Sanofi Genzyme: Speakers Bureau; Oncopeptides: Other: Advisory Board, Speakers Bureau. Lymp:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Vedal:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Nishii:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Avilion:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Papuga:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Nastoupil:ADC Therapeutics: Honoraria; AbbVie: Honoraria; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; Caribou Biosciences: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Regeneron: Honoraria; DeNovo: Honoraria; AstraZeneca: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal